Abstract
Introduction: Non-intensive venetoclax-based regimens are the current standard of treatment for acute myeloid leukemia (AML) in the elderly. Phase 3 VIALE-C (LDAC vs. LDAC+Ven) and VIALE-A (Aza vs. Aza+Ven) trials showed improved median overall survival (OS) from 4.1 to 7.2 months (VIALE-C) and from 9.6 to 14.7 months (VIALE-A), with serious adverse events (SAEs) in 73% low-dose AraC (LDAC), 83% (Aza + Ven), 66% (LDAC + Ven). Quality of the time gained with these regimens is particularly important in this population. Q-TWiST is a useful tool to adjust the OS to quality time without symptoms or toxicity. This kind of analysis has been previously published in newly diagnosed AML in glasdegib + LDAC vs LDAC trials (Solem CT, 2020), and in the CPX-352 vs 7+3 (Cortes JE, 2021) trial. However, venetoclax-based non-intensive regimens have not been analyzed with this method.
Methods: We performed a retrospective, longitudinal analysis of data gathered from a single-center cohort. All newly diagnosed, >60 years old patients with upfront management with venetoclax-based non-intensive chemotherapy combined with LDAC or hypomethylating agents (HMA) at our institution were included. TOX time was defined as the period of time before remission and/or time with serious adverse events (SAE) as defined by Office for Human Research Protections (OHRP) guidance (2007). REL time was defined as time from relapse until death, last follow-up or the start of a second line treatment, TWiST was defined as the time in remission and without SAEs. Q-TWiST analysis was performed as previously described (Solem CT, 2020).
Results: We included 17 patients, 58.8% were male, mean age was 74 (70-78) years. The diagnosis was de novo AML in 52.9%, and secondary AML in 46.9%. European LeukemiaNet (ELN) risk was favorable in 5.8%, intermediate in 11.7% and adverse in 41.1%, 35.2% was unknown, FLT3-ITD mutations were present in 23.5%. 25% of the population had basal ECOG performance status ≥3, HCT-CI score was ≥3 in 47%. Considering the patients with baseline geriatric assessment: 14.2% were frail, 42.8% pre-frail and 42.8% robust.
Venetoclax was combined with azacitidine in 35.2% and LDAC in 64.7%, 11.7% received additional midostaurin. Venetoclax dose was 100 mg/day in combination with strong CYP3A4 inhibitors (mainly itraconazole) in all patients. By last cycle, venetoclax was adjusted to <28 days in 76.6% of patients. Azacitidine dose was adjusted in 60% of patients, and LDAC in 9%.
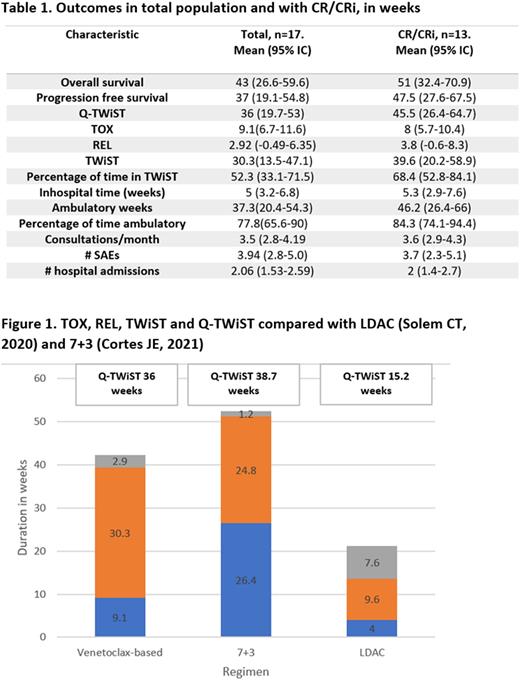
76% of patients achieved complete remission (CR) or complete remission with incomplete hematological recovery (CRi) with the treatment, 23.5% had no response and 28% relapsed. With a mean follow up of 42.4 (25.7-59.1), mean OS was 43.1 (95% IC 26.6-59.6), progression free survival (PFS) 37 (95% IC 19.1-54.8), Q-TWiST 36.3 (95% IC 19.7-53), and TWiST 30.3 (95% IC 13.5-47.1) weeks.
Other variables related to toxicity are shown in table 1. Comparison with previously reported Q-TWiST with LDAC and 7+3 as frontline therapy in the elderly is described in Figure 1.
Conclusions: the Q-TWiST in our unselected population was better than the Q-TWiST described for LDAC arm in the glasdegib trial, this is striking considering the comparison between real world data in a low-income setting with clinical trial population. This suggests that venetoclax-based non intensive chemotherapy not only offers longer OS but also longer quality-adjusted time compared to previous standard to this vulnerable population. When compared to 7+3 regimens the Q-TWiST was slightly shorter in our cohort,probably associated with the inclusion criteria of the trial, including ability to tolerate 7+3 regimen and an age limit of 75 years, which different from our population.
Disclosures
Demichelis:Abbvie: Consultancy, Honoraria; Amgen: Consultancy, Honoraria; Astellas: Consultancy, Honoraria; Teva: Consultancy; Gilead: Consultancy; ASH: Research Funding; Novartis: Consultancy, Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal